MONOTEF
Non-Absorbable Surgical Suture (PTFE)
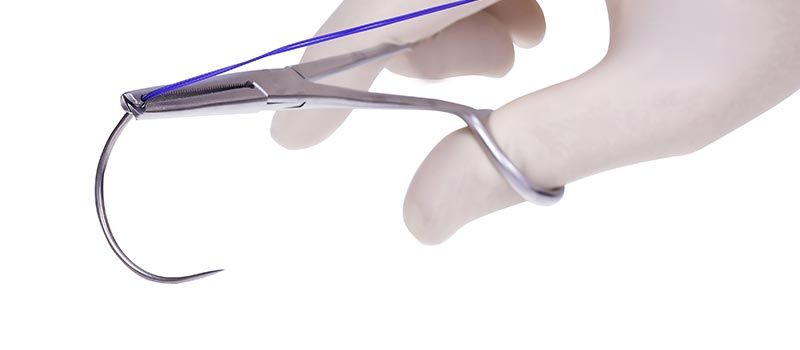
Monotef is a polytetraphloroetylen, non-absorbable, monofilament sterile surgical suture. This suture is manufactured from high-density 100% polytetraphloroetylen that enables a minimum pore size and volume structure, while keeping integrity and tensile strength. This suture is colourless and contains no additive or PFOA.
Monotef suture can be used in general soft tissue closures and bonding including dura mater repair as well as cardiovascular, dental, and general surgery applications. This tools is not indicated for ophthalmic surgery and peripheral neural tissue surgery.
Monotef sutures are manufactured with or without stainless steel needle, between USP 7/0 – USP 0 range, and with various lengths between 45cm to 75cm.